
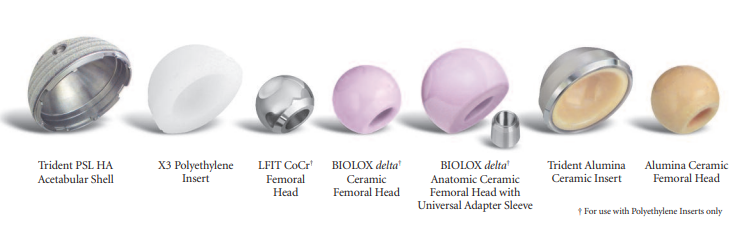
Vendor/Manufacturer: Stryker
Product/Product Line: Trident® Acetabular System
Vendor/Manufacturer Catalog #: 6260-9-122
Global Unique Device (GUD) Primary Device Identifier Number: 07613327013016
Global Unique Device (GUD) Device Description:
V40 Taper LFIT CoCr Heads, LFIT V40 Femoral Head
Global Medical Device Nomenclature (GMDN) Preferred Term Name: Metallic femoral head prosthesis Global Medical Device Nomenclature (GMDN) Definition: An implantable artificial substitute for a diseased or injured femoral head that has an outer surface made of metal [e.g., cobalt-chrome (Co-Cr), titanium (Ti), stainless steel]. It is designed to be attached to the trunnion of a prosthetic femoral stem/shaft or a head/stem adaptor, and to articulate with an acetabulum prosthesis as part of a total hip arthroplasty (THA), or articulate with the natural acetabulum directly or via a bipolar component as part of a partial hip arthroplasty (hemiarthroplasty). The device ranges in form from partially to fully spherical (ball-shaped) and is available in various sizes.
FDA Regulation Medical Specialty: Orthopedic Devices - Prosthetic Devices FDA Regulation Number: 888.3358 FDA Regulation Description Classification Name: Hip joint metal/polymer/metal semi-constrained porous-coated uncemented prosthesis
FDA Classification Product Code(s): JDI, KWL, KWY, KWZ, LPH, LWJ, LZO, MAY, MBL, MEH FDA Classification Product Code Device Name(s): JDI: Prosthesis, hip, semi-constrained, metal/polymer, cemented KWL: Prosthesis, hip, hemi-, femoral, metal KWY: Prosthesis, hip, hemi-, femoral, metal/polymer, cemented or uncemented KWZ: Prosthesis, hip, constrained, cemented or uncemented, metal/polymer LPH: Prosthesis, Hip, Semi-Constrained, Metal/Polymer, Porous Uncemented LWJ: Prosthesis, hip, semi-constrained, metal/polymer, uncemented LZO: Prosthesis, hip, semi-constrained, metal/ceramic/polymer, cemented or non-porous, uncemented MAY: Prosthesis, hip, semi-constrained, metal/ceramic/polymer, cemented or non-porous cemented, osteophilic finish MBL: Prosthesis, hip, semi-constrained, uncemented, metal/polymer, porous MEH: Prosthesis, hip, semi-constrained, uncemented, metal/polymer, non-porous, calicum-phosphate FDA Premarket Submission(s): K061434 , K062419 , K061654 The Trident® large diameter and constrained acetabular inserts and LFIT femoral heads and are SUBSTANTIALLY EQUIVALENT to the following product(s): 1. Sulzer Orthopaedics Inter-Op Durasul Acetabular System
For more information, please visit us at Infonomics.com
Comments